Abstract
Background. T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive disease with few innovative treatment options. This is also contributed by the lack of models capable of capturing the complexity of the tumor and its microenvironment.
Aims. To identify patient-specific vulnerabilities and novel therapeutic strategies in T-ALL and interrogate the mechanisms of the crosstalk between leukemic and stromal elements.
Methods. We established a drug-testing platform using patient-derived-tumor-xenografts (PDTX) and a mixed-culture approach using E4ORF1-transduced endothelial cells (ECs) (Seandel M et al, PNAS 2008) to overcome host-mediated chemoresistance. We performed functional experiments using total and single-cell RNA sequencing.
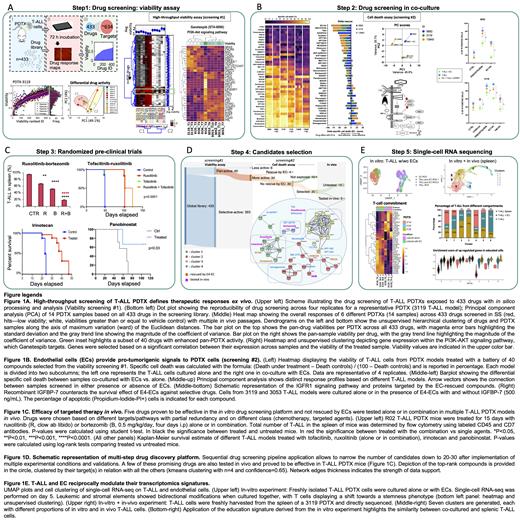
Results. First, we established a battery of 22 T-ALL PDTX models that matched both phenotypically (immune-histochemistry, flow cytometry) and genotypically (TCR rearrangement, transcriptome) with the primary patients' samples. We then challenged these models (n=14 samples belonging to different PDTX and serial passages within each model) with a library of compounds (n=433) targeting redundant proteins (n=634). Unsupervised clustering and Principal Component Analysis (PCA) demonstrated two clusters of T-ALL samples based on differential drug susceptibility. We could at least partially correlate these differences to specific transcriptomic signatures predictive of drug response (Figure 1A).
We then defined a group of pan-active compounds across all models (n=40), which we validated using an independent screening with/without ECs (Figure 1B). We found that ECs counteracted the activity of selected compounds (i.e. TSA, THZ1 and MLN2238). By PCA, we observed distinct response profiles based on different T-ALL models. We vectorized the EC-rescue and found that the direction was the same across all 3 models tested, indicating that it relied on similar mechanisms regardless of model identity. Based on the known role of IGF1-IGFR1 as a supportive EC-rescue axis (Medyouf H et al, J Exp Med 2011), we performed the same screening with/without recombinant IGFBP-7 (500 ng/mL), a decoy IGF1 molecule. Remarkably, IGFBP-7 completely or partially abrogated the EC-mediated rescue of selected compounds [enzastaurin (PKC-β inhibitor), SC144 (GP130 inhibitor), CHIR124 (Chk1 inhibitor) and YM155 (Survivin inhibitor)] (Figure 1B). Drugs not rescued by ECs (n=30) were considered positive hits and 5 of them (ruxolitinib, tofacitinib, panobinostat, bortezomib, irinotecan) ultimately proved to be effective in vivo in randomized pre-clinical trials either alone or in combination (Figure 1C). Our stepwise endothelial-leukemia platform led to the discovery of "public" and "private" vulnerabilities and the proof-of-principle of prediction-guided in vivo pre-clinical trials. We propose a list of compounds that could be readily translated into T-ALL clinical trials (Figure 1D). We finally proved the validity of our platform using other disease models (i.e. B and T-lymphoma PDTXs).
Mechanistically, at single-cell resolution, in vitro interacting T-ALL cells and ECs underwent reciprocal transcriptome changes, with T-ALL shifting towards stemness/undifferentiation and ECs towards tumor-ECs (TECs) phenotypes. Furthermore, in vitro EC-educated T-ALL cells mimicked distinct T-ALL subsets of the leukemic spleen of corresponding PDTX mice (Figure 1E).
Conclusions. These data demonstrate that our EC-T-ALL culture system simulates in vivo conditions, offering a robust platform to study drug response, leukemia-host interactions and cell plasticity. This approach will improve the pre-clinical predictability of novel drugs/combinations for T-ALL, as well as for other hematologic malignancies, and propel the development of patient-tailored treatments.
Melnick: Janssen Pharmaceuticals: Research Funding; Sanofi: Research Funding; Daiichi Sankyo: Research Funding; Epizyme: Consultancy; Constellation: Consultancy; KDAC Pharma: Membership on an entity's Board of Directors or advisory committees. Elemento: AstraZeneca: Research Funding; Freenome: Consultancy, Other: Current equity holder in a privately-held company; Volastra Therapeutics: Consultancy, Other: Current equity holder, Research Funding; Champions Oncology: Consultancy; Owkin: Consultancy, Other: Current equity holder; One Three Biotech: Consultancy, Other: Current equity holder; Eli Lilly: Research Funding; Johnson and Johnson: Research Funding; Janssen: Research Funding. Chiaretti: amgen: Consultancy; pfizer: Consultancy; novartis: Consultancy; Incyte: Consultancy. Cerchietti: Celgene: Research Funding; Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal